Researchers in Germany have designed an organic reagent capable of inserting a single carbon atom into existing molecules – one of three group transfer reactions supported by the new compound. The designed reagent is easy to make and can become a powerful addition to the skeleton editing toolbox.
Insertions of carbon atoms are important transformations in organic chemistry, and homologations such as the Wittig reaction are staples of undergraduate chemistry. These processes typically use a dipolar reagent called a ylide which contains a (formally) negatively charged carbon atom bonded to a (formally) positively charged heteroatom which is cleaved during the reaction. However, while this chemistry is well established, many ylide reagents are synthesized using explosive azides and are unstable at room temperature, limiting both the practicality and scope of these techniques.
With the aim of expanding the potential of carbon transfer reactions, Max Hansmann and his team at the Technical University of Dortmund in Germany attempted to design an alternative ylide reagent. They envisioned a central carbon atom surrounded by easily separable triphenylphosphine and diazo groups. “It’s very well known that you can cleave CP and CN bonds with this moiety, so it would be the ideal reagent to transfer a carbon atom,” he says.
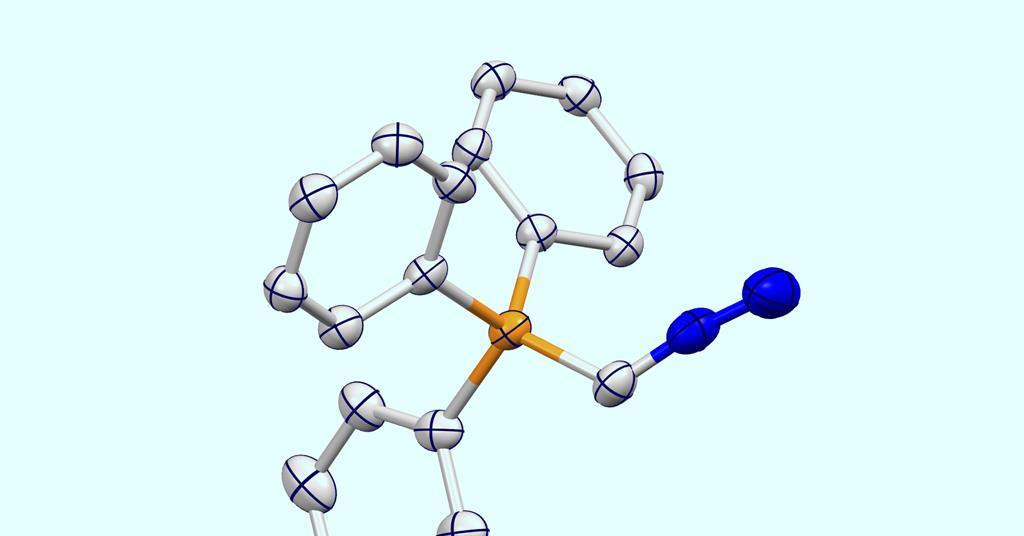
With safety in mind, the team’s synthesis deliberately avoids problematic azide reagents, instead gently heating a diphosphorane precursor with nitrous oxide to prepare the proposed ylide. The resulting solid reagent exhibits high thermal stability, and its cumulene-like structure – containing three consecutive double bonds – was confirmed by x-ray analysis.
CP and CN bonds can be broken in subsequent reactions, and the team began to explore how to direct the various possible group transfer processes. “You control the reactivity through the substrate you provide,” explains Hansmann. ‘An α,β-unsaturated compound will usually react through a [3+2] cycloaddition, transferring CN2 to give a pyrazole. An electrophilic species such as carbon monoxide undergoes an unusual substitution mechanism involving an exchange of transition metal ligands, dissociating N2 and the transfer of CPPh3.’
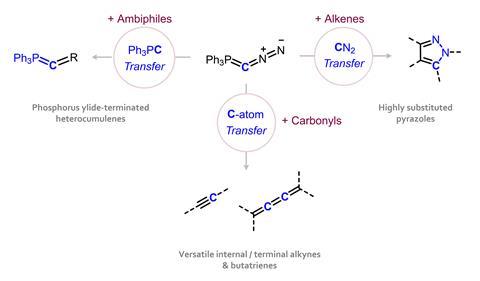
More importantly, by cleaving both triphenylphosphine and diazo groups, the reagent can transfer a single carbon atom to a carbonyl reagent, ultimately generating alkyne and butatriene products. “The first step is very similar to the Wittig-a reaction [2+2] cycloaddition with carbonyl. Then you cleave the triphenylphosphine to generate a diazoalkene, cleave the N2 to get a vinylidene and then you have different paths,’ he explains. ‘1-2 migration leads to the alkyne, or you dimerize and access the butatriene – it depends on the carbonyl.’ For Hansmann, this is the most valuable reactivity for the organic chemistry community—providing a new, facile route to these important functional groups and overcoming the limitations of traditional ylide reagents.
Initially the team focused on creating this carbon transfer reactivity for relatively simple substrates, but now they are exploring its potential for larger and more complex systems. “There may be applications in editing the skeleton, for example, expanding the rings and inserting a carbon atom at a later stage,” says Hansmann. “It could also be interesting for the core group community as you can essentially play with carbon coordination complexes.”
For Mark Levin, an organic chemist at the University of Chicago in the US, the ylide’s potential as a tool in skeleton editing is the most exciting aspect of the work. “Reagent design is an underappreciated aspect of progress in organic chemistry and [in skeletal editing] has a unique set of challenges that further complicate reagent design,” he says. “I think this reagent is going to be quite popular going forward – it’s such a simple synthesis from carbodiphosphorane and nitrous oxide, and the reactivity they’ve already shown is quite interesting.”