In a suit filed against CVS, Walgreens, GE Healthcare and many other medical technology companies and sellers of pulse oximeters – devices that use light to measure oxygen saturation in the blood – Medtronic has been dismissed, the company said in a statement Monday.
Meanwhile, the U.S. Food and Drug Administration has been working to update its 2013 guidance on pulse oximetry submissions; publication and public comment have been pushed to fiscal year 2025.
WHY IT MATTERS
Medtronic, a medical device and technology company, was one of many defendants named in a lawsuit the Roots Community Health Center filed in Alameda County Superior Court last year.
Slammed during the COVID-19 epidemic, when the death rate among Black Americans was disproportionately high, Roots said in its announcement about the lawsuit that it had relied on pulse oximeters in its decisions to provide oxygen therapy to the swelling lines of patients coming to it for medical care.
Roots asked the court to halt sales of “unreliable pulse oximeters or require manufacturers and distributors of the flawed devices to warn purchasers of their products’ dangerous shortcomings.”
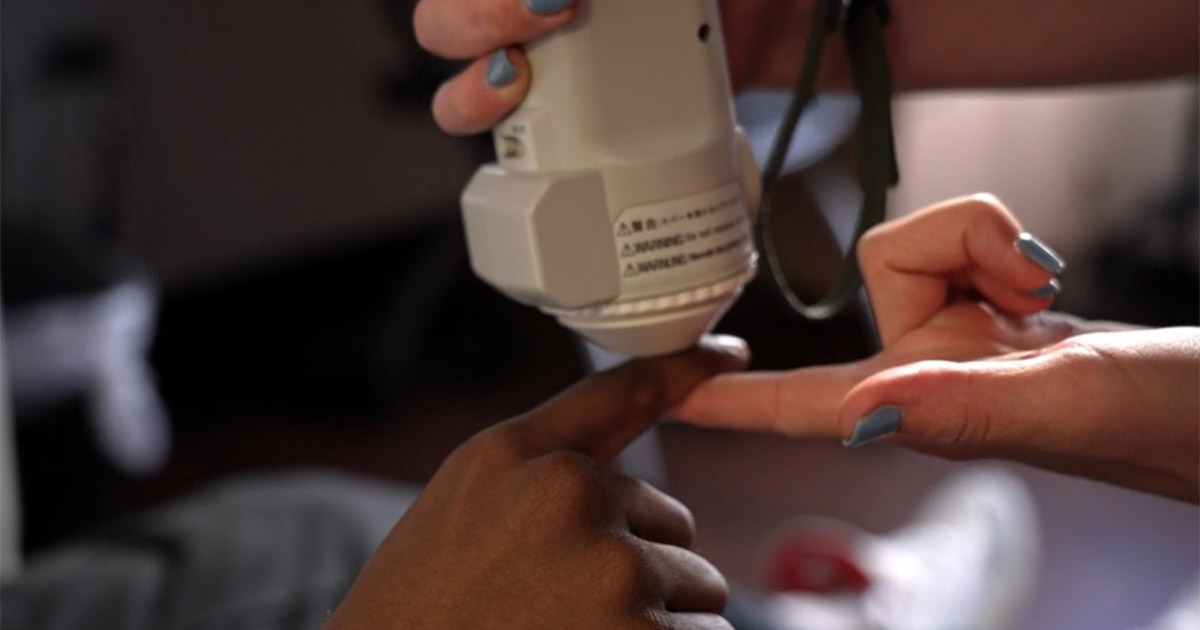
“At least one of Medtronic/Covidien’s pulse oximeters that is cleared by the FDA notes in the manual that accompanies the pulse oximeter that dark skin pigment can cause inaccurate measurements,” Roots said in the filing.
“The manual does not explain that measurements in people with darker skin are skewed towards showing that people with darker skin have more oxygen than they do in reality, nor that dark skin pigmentation can interact with other conditions to cause or exacerbate inaccurate measurements.”
While Roots also alleged that Medtronic and other companies “knew or should have known” that statements not cleared by the FDA “are untrue or misleading and would deceive or would be likely to deceive consumers,” at this time, “Roots agreed to dismiss claims against Medtronic,” a spokesperson for the company told Healthcare IT News by email.
The spokesperson said that Medtronic has been recruiting more “diverse participants” for its clinical studies, is educating medical providers on the proper use of pulse oximetry and has invested in technology improvements over the last two years.
THE LARGER TREND
Research published in the New England Journal of Medicine in late 2020 found pulse oximeters could be less accurate for Black patients and since that time many pulse oximeter critics say speed to market led to the medical community and regulators overlooking how skin tone can affect a pulse ox reading.
According to critics at the Johns Hopkins School of Medicine in Health Policy and Management at the Bloomberg School, FDA’s standards for pulse oximeters “essentially ignored” the functional racial disparity found to be ubiquitous among light-sensing devices. The health policy academics argued in magazine articles and in its podcast series earlier this year that eliminating pulse oximetry bias requires decisive regulatory action.
The FDA’s Center for Devices and Radiological Health told Healthcare IT News in July that revising its 2013 pulse oximeter guidance is listed on the CDRH Guidance Agenda as a priority with the intent to publish in FY 2024, which ended September 30.
“The FDA has been engaging stakeholders, gathering input from ongoing clinical research to help inform our decisions and evaluating all available information pertaining to factors that may affect pulse oximeter accuracy and performance,” the spokesperson said at the time.
However, two months after being grilled by Congress over medical device missteps along with his colleagues, CDRH director Dr. Jeff Shuren stepped down in late July.
Then last week, the FDA announced that Dr. Michelle Tarver, an ophthalmologist with a doctorate in epidemiology who has held various leadership positions at the FDA as a medical device regulator, would replace Shuren as permanent CDRH director.
We reached out to FDA for an update on the dangling status of updated pulse oximeter guidance and asked for Tarver’s POV on pulse oximeters.
“The 2024 CDRH guidance agenda included guidance documents CDRH intended to publish in fiscal year 2024,” FDA’s spokesperson explained by email Wednesday. “However, the center is neither bound by this list of topics nor required to issue every guidance document on the list.”
The spokesperson added, “The FDA has been working diligently to update its 2013 final guidance on pulse oximeters and is committed to issuing these new draft recommendations as expeditiously as possible.”
“Updating this guidance is a priority for CDRH.”
We’ve reached out to Roots about the agreement with Medtronic and the status of FDA’s pulse oximeter submission guidance and will update this story if there is a statement.
ON THE RECORD
“Medtronic is pleased to announce that Roots Community Health has agreed to dismiss claims against the company related to pulse oximeters,” Medtronic said in a statement.
“Medtronic looks forward to working with Roots, the FDA and other key stakeholders to ensure health equity can be achieved through technology, educational efforts and partnerships.”
Andrea Fox is senior editor of Healthcare IT News.
Email: afox@himss.org
Healthcare IT News is a HIMSS Media publication.