 Sanjeev Panchal, Country President & Managing Director for AstraZeneca India; Photo: Selvaprakash Lakshmanan for Forbes India
Sanjeev Panchal, Country President & Managing Director for AstraZeneca India; Photo: Selvaprakash Lakshmanan for Forbes India
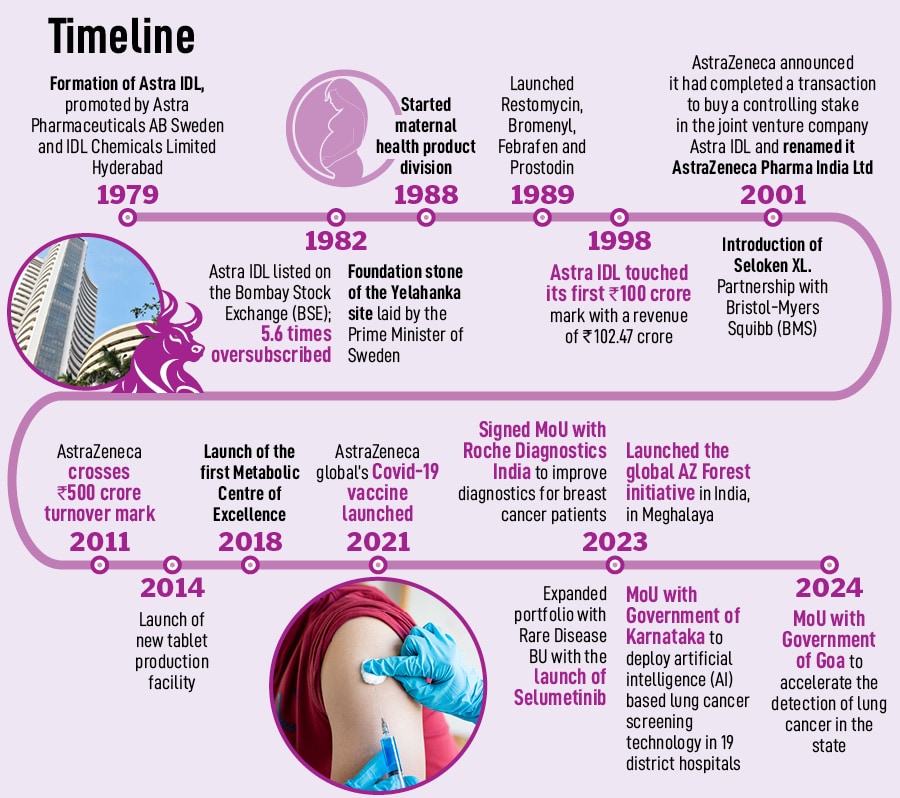
AstraZeneca has had a strong presence in the Indian market for nearly 45 years, introducing over 50 innovative medicines to date. In India, the pharma major’s focus remains on areas including biopharmaceuticals (comprising cardiovascular, renal metabolism and respiratory), oncology, immune therapies and rare disease. “We want to bring innovative medicines to India, faster,” says Sanjeev Panchal, country president and managing director, AstraZeneca. Today, the company is more committed than ever to accelerating the development and delivery of breakthrough therapies, with a focus to fast-track its innovative drug pipeline in India.
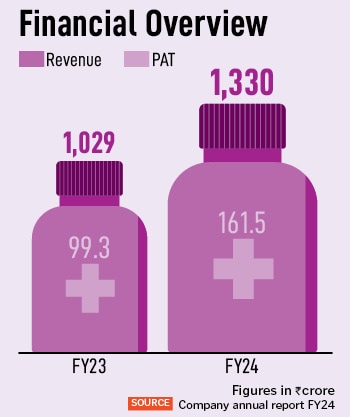
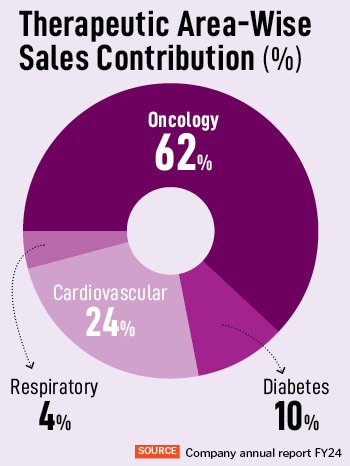 AstraZeneca India announced its Q2 FY24 results today, touching a 31 percent increase in revenue from operations compared to the same period last year. For Q2 FY24, its revenue from operations is Rs 408 crore. The company has achieved a year-on-year growth of 29 percent, with Rs 1,330 crore in revenues in FY24. “We are strengthening our footprint in India, for India and the world,” says Panchal, who has been with the organisation for close to 21 years, in various roles and across geographies. Of this, oncology accounts for 62 percent of the company’s total revenue, and has also seen the highest revenue growth of 43 percent. “These key therapy areas are pivotal for our growth, through the innovation strategy,” adds Panchal.
AstraZeneca India announced its Q2 FY24 results today, touching a 31 percent increase in revenue from operations compared to the same period last year. For Q2 FY24, its revenue from operations is Rs 408 crore. The company has achieved a year-on-year growth of 29 percent, with Rs 1,330 crore in revenues in FY24. “We are strengthening our footprint in India, for India and the world,” says Panchal, who has been with the organisation for close to 21 years, in various roles and across geographies. Of this, oncology accounts for 62 percent of the company’s total revenue, and has also seen the highest revenue growth of 43 percent. “These key therapy areas are pivotal for our growth, through the innovation strategy,” adds Panchal.
In November 2023, AstraZeneca Pharma India Ltd announced its exit from manufacturing at its plant in Bengaluru, as part of a global strategic review. “This decision was also made in line with our focus on specialist disease areas, and our efforts to bring medicines faster to India via imports,” he adds. AstraZeneca has decided to import specialist medicines to India, and simultaneously partner locally to expand access where appropriate.
Focusing on innovation
AstraZeneca’s mission is to make cancer as manageable as diabetes. “We are leading a revolution in oncology to redefine cancer care. We are following the science to understand cancer and all its complexities to discover, develop and deliver life-changing treatments and increase the potential to cure,” says Anil Kukreja, vice president, medical and regulatory, AstraZeneca Pharma India.
Also read: Global pharma: Focus on core business, pure-play innovation
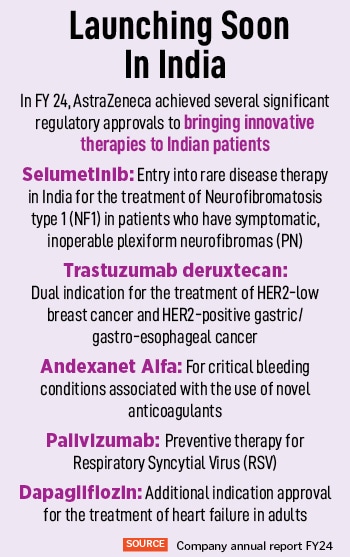
With oncology, the company has medicines focussed on different types of cancer, including lung cancer, breast cancer and gastrointestinal (GI) cancer. “We are also expanding, in terms of indications. For instance, earlier we were looking at late-stage lung cancer, but now we are expanding to new medicines or indications that can be used in early stages,” says Panchal. Earlier this year, the company launched Trastuzumab deruxtecan, a therapy tailored for the treatment of adult patients with unresectable or metastatic HER2 positive breast cancer for those who have previously received an anti-HER2 regimen.
 Within oncology, the pharma giant is working on antibody drug conjugates (ADC), tumour drivers and resistance therapies, and immuno-oncology (IO). “ADCs are highly targeted and have a cell-specific mechanism to reduce side effects for those being treated. We are also developing the next wave of IO therapies that aim to empower the immune system, to more effectively recognise and kill cancer cells and to overcome the immunosuppressive mechanisms that cancers frequently develop as they evolve,” explains Kukreja.
Within oncology, the pharma giant is working on antibody drug conjugates (ADC), tumour drivers and resistance therapies, and immuno-oncology (IO). “ADCs are highly targeted and have a cell-specific mechanism to reduce side effects for those being treated. We are also developing the next wave of IO therapies that aim to empower the immune system, to more effectively recognise and kill cancer cells and to overcome the immunosuppressive mechanisms that cancers frequently develop as they evolve,” explains Kukreja.
For biopharmaceuticals, AstraZeneca has received approvals for essential treatments such as a triple inhalation aerosol for managing chronic obstructive pulmonary disease (COPD) delivered through a device, Palivizumab, for preventing respiratory syncytial virus (RSV) for premature and high-risk infants, and Andexanet Alfa for managing life-threatening bleeding linked to Factor Xa (FXa) inhibitors. “I think what is important for me is the unmet need in the disease areas and where we can make the most meaningful difference to patients,” says Panchal.
On the immunology front, AstraZeneca and Sanofi’s Beyfortus (nirsevimab), a long-acting monoclonal antibody, has been approved in Japan for the prophylaxis of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in all neonates, infants and children entering their first RSV season, and the prevention of RSV LRTD in neonates, infants and children at risk of serious RSV infection entering their first or second RSV season. AstraZeneca India is planning to bring this drug to India as well.
The strategy for AstraZeneca is to recognise the unmet needs in disease areas, and to make the most meaningful difference to patients. Panchal adds, “For instance, rare diseases. We brought a medicine for Neurofibromatosis type 1 [NF1] last year. We want to continue focusing on such gaps,” he remarks. With this in mind, the company is simultaneously participating in more global clinical trials across its therapy areas.
“From a topline perspective AstraZeneca is doing well; they are the fastest growing pharma MNC in India, clocking a 30 percent topline growth,” says Vishal Manchanda, senior vice president, Institutional Research, Systematix Group.
The target, according to Panchal is to touch 15 new launches—a combination of new indications and drugs—by 2025. “We want to be leading in specialist diseases and truly transform patient outcomes, by focusing on early diagnosis. And of these 15, we have already got nine new approvals, so we are on track.”
Accessibility and affordability
While AstraZeneca has been bringing many innovative medicines to India, the challenge of accessibility and affordability, remains. To solve for this, the company is working with various state governments as well as many Indian players to reach the masses. Panchal says, “As the world is moving towards targeted therapy for cancer, we are prioritising work around early detection of lung cancer, breast cancer and GI cancer.”
For instance, the company has collaborated with Qure.ai, an organisation developing deep learning algorithms for radiology image interpretation for lung cancer. “About 85 percent of lung cancer cases are diagnosed late. This is because when the nodule is small in the lungs, the patient has no symptoms. By the time, symptoms are visible it is too late and there is only a 5 percent survival for late-stage detection of lung cancer,” explains Prashant Warier, co-founder and CEO, Qure.ai. Usually, lung cancer is detected via targeted CT screenings. But often the issue is that not enough people get these screenings or there isn’t enough capacity to accommodate people especially for the free screenings. “But with Qure.ai’s solution, doctors can screen patients for lung cancer through X-rays, ensuring that at-risk patients gain access to necessary therapies faster,” adds Warier.
With support of the Karnataka government, Qure.ai has deployed this solution in 19 district hospitals across the state. “Along with AstraZeneca, we have now scaled up to more than 30 countries and the company has committed to processing 5 million scans through Qure.ai’s technology by 2025,” says Warier.
Recently, the company signed a memorandum of understanding (MoU) with the Government of Goa to accelerate lung cancer detection, emphasising early detection and intervention in combating lung diseases. Qure.ai also signed an MoU with Rajiv Gandhi Cancer Institute and Research Centre (RGCI&RC), Delhi, to establish a centre of excellence for subsidised, high-quality next-generation sequencing (NGS) molecular panel testing for individuals diagnosed with lung cancer. Additionally, the pharma company has partnered with Roche Diagnostics India to enhance diagnostics for breast cancer patients, focusing on streamlining HER2 diagnostics with advancements in the field.
 Apart from innovator drugs, AstraZeneca also has a bunch of drugs that cater to the masses. For these, AstraZeneca entered into an agreement with Mankind Pharma Limited for exclusive distribution of its asthma drug brand Symbicort (budesonide and formoterol fumarate dihydrate), an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination, in India. “While we focus on specialist medicines, we also believe there are some drugs that need to reach a higher number of patients. Hence, partnering with a player like Mankind expands access,” says Panchal. The partnership aims to accelerate access and maximise the potential of the asthma drug and the Turbuhaler, a device to deliver a higher proportion of respirable particles.
Apart from innovator drugs, AstraZeneca also has a bunch of drugs that cater to the masses. For these, AstraZeneca entered into an agreement with Mankind Pharma Limited for exclusive distribution of its asthma drug brand Symbicort (budesonide and formoterol fumarate dihydrate), an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination, in India. “While we focus on specialist medicines, we also believe there are some drugs that need to reach a higher number of patients. Hence, partnering with a player like Mankind expands access,” says Panchal. The partnership aims to accelerate access and maximise the potential of the asthma drug and the Turbuhaler, a device to deliver a higher proportion of respirable particles.
“Most MNCs have been following this trend—this partnership makes sense because AstraZeneca has limited bandwidth in India, and can hardly do justice to a product like Symbicort, which is a mass-market product. It would need a large sales force to be effective. Hence, it is appropriate to leverage Mankind’s reach for such products; it ends up being a win-win for both,” explains Manchanda.
The Indian pharmaceutical industry must prioritise innovation to remain competitive and meet evolving health care needs. Recently, the government launched an R&D policy to encourage local innovation. “We are trying to make our medicines more affordable for patients. In this also, the government announced GST reduction. Lastly, a clinical trial waiver was also announced, under which if a drug is approved in six countries, there will be a clinical trial waiver. So, while there are challenges, we are seeing progress, which gives the global company confidence to invest more in a market like India,” explains Panchal.
AstraZeneca’s tech play
The company has two entities, one of which is listed—AstraZeneca Pharma India Limited—and the other AstraZeneca India Private Limited, which caters to IT services, global business services and R&D capabilities.
Earlier this year, AstraZeneca announced its plans to invest Rs 250 crore to expand its Global Innovation and Technology Centre (GITC) in India, creating around 1,300 highly skilled roles by 2025. “India has become AstraZeneca’s largest footprint for technology and GBS business in the world,” adds Panchal.
When AstraZeneca established the GITC in Chennai in 2014, it was mostly an IT support services centre with 300 people, but now it has evolved into a global hub of innovation, with over 50 percent of the global IT staff located here. “In alignment with our mission of using science to improve patients’ lives, we leverage data analytics, artificial intelligence and machine learning to drive transformation in health care,” remarks Panchal. India is the nerve centre of technology and innovation for AstraZeneca, leading the company’s digital agenda worldwide.
As AstraZeneca celebrates 45 years of its presence in India, Panchal reckons, “Our purpose is to push the boundaries of science, to deliver life-changing medicine. We want to bring the science to the patient, and transform the future of health care.”
