FDA updates guidance on pulse ox devices used in healthcare
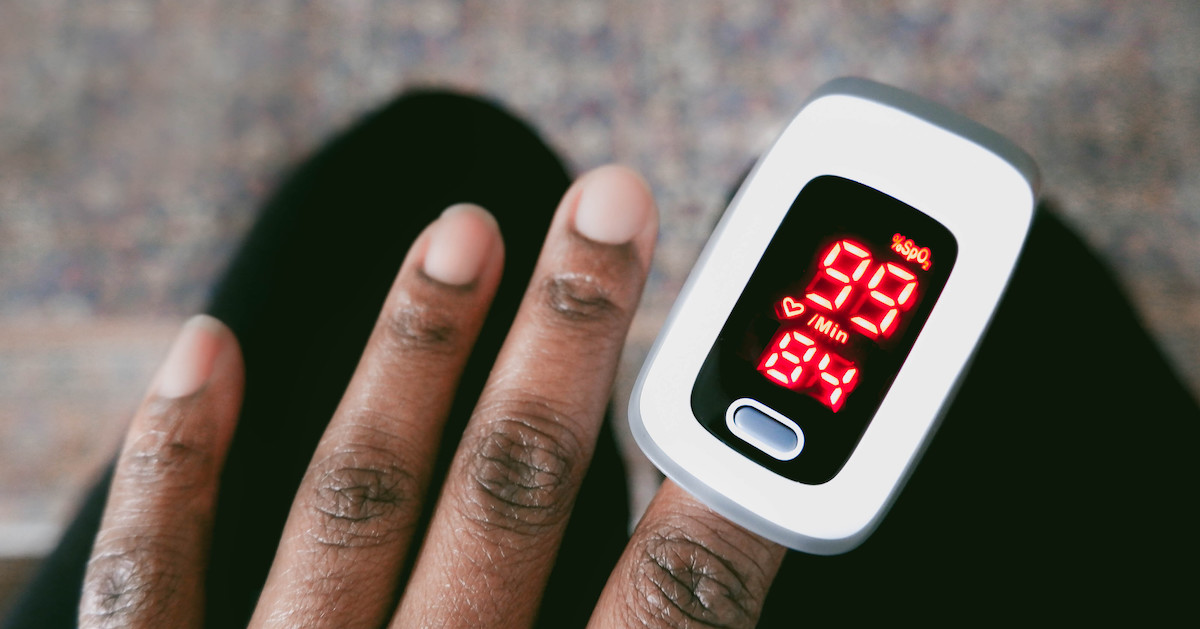
The U.S. Food and Drug Administration is seeking public comment on new recommendations for nonclinical and clinical performance testing to support premarket submissions for pulse oximeters for medical purposes. These include devices with a function that estimates the amount of oxygen in arterial blood and pulse rate. WHY IT MATTERS During the COVID-19 pandemic, medical … Read more