
John Dawber, Corporate VP and MD, GBS, Novo Nordisk
Image: Selvaprakash Lakshmanan for Forbes India
An Englishman running an Indian GCC for a Danish multinational drug company is probably a great example of how far some of these centres have come, courtesy globalisation.
John Dawber will tell you, if you ask him, he’s only worked for two companies, and the second one is Novo Nordisk, which he joined on the advice of a doctor he respected. He’s currently corporate vice president and managing director for global business services (GBS). Based in Bengaluru, Dawber leads the company’s 4,400 GBS employees.
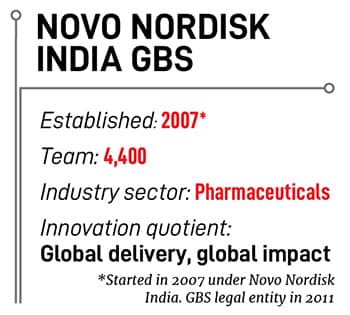
What is today a full-fledged GBS, as companies such as Novo Nordisk call their GCCs, started 17 years ago, in 2007, to tap the-then emerging opportunity of saving costs via what were called shared services centres. Therefore, initially it was just about offshoring some low-value transactional work in finance and a bit of IT.
“But there was also a sort of curiosity about that as to what could be done,” Dawber tells Forbes India. One of the first such experiments was a small group set up in Bengaluru by Novo Nordisk’s legal patents organisation, to work on “competitive intelligence” in terms of legal and patents work.
 Back then, the primary driver was cost arbitrage, like for many other companies, Dawber says. However, by 2012 or so, as Novo Nordisk itself grew, with an expanding portfolio of drugs, it became apparent that it could go beyond finance and IT into technology and medical technology. In particular, “pharmaceutical skills were something that we could deliver at scale”.
Back then, the primary driver was cost arbitrage, like for many other companies, Dawber says. However, by 2012 or so, as Novo Nordisk itself grew, with an expanding portfolio of drugs, it became apparent that it could go beyond finance and IT into technology and medical technology. In particular, “pharmaceutical skills were something that we could deliver at scale”.
The 4,400 staff at Novo Nordisk’s GBS in India span 17 different functions, which pretty much represents the whole pharma value chain, he says, with only the laboratories and factories not present in India. Those are expected to remain in Denmark.
“Everything in between is pretty much represented here,” he says. This includes research, early development, clinical trials, data management, regulatory, global medical affairs, safety, pharmacovigilance, medical writing, commercial, and global marketing. And, of course, finance, global HR and IT.
Even what once required a lab can now be done on a computer and that’s bringing its own opportunity to push the boundaries on what can be delivered from India, he says. For example, a small team of eight specialists is already working on related data sciences work.
In the area of biostatistics or statistical analysis of medical and clinical data from clinical trials and so on, Novo Nordisk has four global centres—two in Denmark, one in Boston, in the US, and the fourth in Bengaluru. And there is no differentiation between the type of work that’s done in Boston versus Bengaluru, Dawber says.
Also read: We will be building AI from India: Meltwater’s Aditya Jami
Rather, the different locations in different time zones allow the company to do this work round the clock.
And despite the complex nature of drug discovery and getting medicines from the lab to markets globally, Novo Nordisk is also already collaborating with startups in India, and in the R&D space.
The first example here is in the area of clinical reporting. A small innovation team at Novo Nordisk’s Bengaluru GBS collaborated with a startup to develop a solution that reduced time needed for quality checks of documents authored by medical doctors and PhDs from 40 hours to just one hour—using an AI solution from the startup. Dawber didn’t want to name the startup.
“We’re now scaling that to other areas within the business,” Dawber says, such as legal and contracts, across the company. “It was born in Bengaluru and it’s now in use in more places.”

Another example is a more work-in-progress effort, to build a solution using generative AI to make producing communications collateral a breeze for the company’s scientists and others. This is also in collaboration with a different startup, and the solution is currently in a pilot phase.
“The time is ripe. We’ve got a level of maturity here and we can optimise and automate. Then we can look for really interesting work to be done here to impact Novo Nordisk globally.”
When asked if Bengaluru played any role in developing Ozempic, Dawber would say no more than that the GBS did its bit.
Over the next 12 months, the GBS will probably exceed 5,000 employees, he says. More importantly, he also wants to foster “an environment where people can speak up, where people can do good cross-functional collaboration”, he says.
(This story appears in the 21 February, 2025 issue
of Forbes India. To visit our Archives, click here.)

 Back then, the primary driver was cost arbitrage, like for many other companies, Dawber says. However, by 2012 or so, as Novo Nordisk itself grew, with an expanding portfolio of drugs, it became apparent that it could go beyond finance and IT into technology and medical technology. In particular, “pharmaceutical skills were something that we could deliver at scale”.
Back then, the primary driver was cost arbitrage, like for many other companies, Dawber says. However, by 2012 or so, as Novo Nordisk itself grew, with an expanding portfolio of drugs, it became apparent that it could go beyond finance and IT into technology and medical technology. In particular, “pharmaceutical skills were something that we could deliver at scale”.
